|
Anapafseos 5 Agios Nikolaos 72100,Crete,Greece,00302841026182,00306932607174,alsfakia@gmail.com
Blog Archive
- ► 2022 (3010)
- ► 2021 (9899)
-
▼
2020
(4138)
-
▼
November
(1979)
-
▼
Nov 11
(96)
- GBE attenuates arsenite‐induced hepatotoxicity by ...
- GDF11 restricts aberrant lipogenesis and changes i...
- Insights into the regulatory role and clinical rel...
- The potential use of theranostic bacteria in cancer
- Cancers, Vol. 12, Pages 3329: Current Advances and...
- Cancers, Vol. 12, Pages 3330: Prognostic Factors I...
- Cancers, Vol. 12, Pages 3331: Cancer Extracellular...
- Cancers, Vol. 12, Pages 3332: The Potential of Lon...
- The p.Ser64Leu and p,Pro104Leu missense variants o...
- Novel homozygous truncating variants in ZMYND15 ca...
- A Novel Variant in COX16 Causes Cytochrome c Oxida...
- Aberrant COL11A1 splicing causes prelingual autoso...
- Multiomic analysis elucidates Complex I deficiency...
- A Functional Variant on 20q13.33 Related to Glioma...
- The future of cancer screening
- What matters most: Randomized controlled trial of ...
- Indoor tanning exposure in association with multip...
- Associations of baseline patient‐reported outcomes...
- A multi‐institutional phase 2 trial of stereotacti...
- Tiotropium/Olodaterol Delays Clinically Important ...
- Impact of gastrointestinal symptoms on quality of ...
- Aggravation of Food Allergy by Skin Sensitization ...
- Cancers, Vol. 12, Pages 3336: The Role of Oxidativ...
- Cancers, Vol. 12, Pages 3333: Increased PARP Activ...
- Cancers, Vol. 12, Pages 3335: A Comparative Oncolo...
- Liver tumor F-18 FDG-PET before and immediately af...
- A 5-year-old boy with acute neurological disorder ...
- Antibiotics, Vol. 9, Pages 798: Antimicrobial Resi...
- Grandparental dietary provision, feeding practices...
- Cancers, Vol. 12, Pages 3339: Modulation of de Nov...
- Cancers, Vol. 12, Pages 3338: Hydrogen Sulfide-Evo...
- JPM, Vol. 10, Pages 220: Impact of Single-Nucleoti...
- Febuxostat Attenuates the Induction of Vascular Ce...
- Bacterial etiology of sputum from tuberculosis sus...
- Radiological manifestations of thoracic hydatid cy...
- Contralateral Effects of Unilateral Strength and S...
- Treatment of cartilage defects by Low-intensity pu...
- Brain donation
- JFB, Vol. 11, Pages 81: Novel Biofuel Cell Using H...
- Combination of lysine‐specific demethylase 6A (KDM...
- Functional mechanism and clinical implications of ...
- Rapid and Scalable Profiling of Nascent RNA with f...
- A Switch in p53 Dynamics Marks Cells That Escape f...
- Dopamine Inputs from the Ventral Tegmental Area in...
- Generation of universal and hypoimmunogenic human ...
- The roles and functions of Paneth cells in Crohn’s...
- Biosensors, Vol. 10, Pages 173: Electromagnetic Pi...
- Factors Influencing Total Serum IgE in Adults: The...
- Intralymphatic Administration of Metagonimus yokog...
- J. Intell., Vol. 8, Pages 38: A Reappraisal of the...
- Coronary anatomy and comorbidities impact on elect...
- Transcatheter mitral valve thrombosis: A case repo...
- VersaCross transseptal system for transcatheter mi...
- ' ... sciens quia melior est misericordia tua supe...
- The emerging landscape of nanotheranostic-based di...
- JPM, Vol. 10, Pages 221: Fluid Candidate Biomarker...
- Ureterovesical Junction Deformation During Urine S...
- Altered stress field of the human lens capsule aft...
- Hospital bed height influences biomechanics during...
- The effects of exterior boundary conditions on a i...
- Modified SHI‐medium supports growth of a disease‐s...
- Use of Patient-Reported Symptoms from an Online Sy...
- Functional and clinical significance of ROR1 in lu...
- Feasibility of improving patient’s safety with in ...
- EyeDose: An open-source tool for using published M...
- Evidence for interleukin 17 involvement in severe ...
- Non-coding RNA derived from extracellular vesicles...
- Mitochondrial rewiring through mitophagy and mitoc...
- PRMT6 deficiency induces autophagy in hostile micr...
- Modification of diet, exercise and lifestyle (MODE...
- Awake prone positioning of hypoxaemic patients
- Development of a risk prediction model of potentia...
- Development and validity testing of the Adolescent...
- Investigating a new tablet-based telerehabilitatio...
- Discontinuing antidepressant medication after mind...
- Effectiveness of deep electroacupuncture with stro...
- TThe Modification of Diet, Exercise and Lifestyle ...
- Assessing the benefits on quality of life of a mul...
- Current status of radioligand therapy and positron...
- [ASAP] Microenvironment-Triggered Degradable Hydro...
- Flow‐diverting stents in the treatment of peripher...
- Usefulness of updated logistic clinical SYNTAX sco...
- TDP-43 proteinopathies: a new wave of neurodegener...
- Kinetic Modelling and Test–Retest Reproducibility ...
- Aberrant ALOX5 Activation Correlates with HER2 Sta...
- Octreotide Infusion for the Treatment of Congenita...
- Three-Decade Evaluation of Cerebrospinal Fluid Pre...
- Erosion Infiltration in the Management of Molar-In...
- sEMG-Based Neural Network Prediction Model Selecti...
- Establishment and Validation of a Prognostic Risk ...
- The Composition of Gut Microbiota in Patients Bear...
- Use of Argon Plasma Coagulation and Endoscopic Hem...
- The Regenerative Potential of Donkey and Human Mil...
- Biochanin A Mitigates Atherosclerosis by Inhibitin...
- Some Common SNPs of the T-Cell Homeostasis-Related...
- Early Electroacupuncture Extends the rtPA Time Win...
-
▼
Nov 11
(96)
-
▼
November
(1979)
- ► 2019 (2429)
Αλέξανδρος Γ. Σφακιανάκης
Wednesday, November 11, 2020
GBE attenuates arsenite‐induced hepatotoxicity by regulating E2F1‐autophagy‐E2F7a pathway and restoring lysosomal activity
GDF11 restricts aberrant lipogenesis and changes in mitochondrial structure and function in human hepatocellular carcinoma cells
|
Insights into the regulatory role and clinical relevance of mediator subunit, MED12, in human diseases
|
The potential use of theranostic bacteria in cancer
|
Cancers, Vol. 12, Pages 3329: Current Advances and Hurdles in Chimeric Antigen Receptor Technology
|
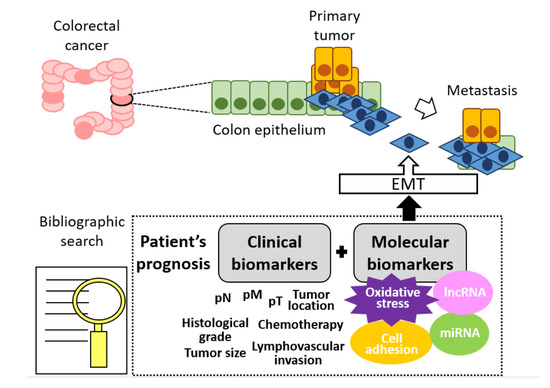
Cancers, Vol. 12, Pages 3330: Prognostic Factors Involved in the Epithelial–Mesenchymal Transition Process in Colorectal Cancer Have a Preponderant Role in Oxidative Stress: A Systematic Review and Meta-Analysis
|
Cancers, Vol. 12, Pages 3331: Cancer Extracellular Matrix Proteins Regulate Tumour Immunity
|
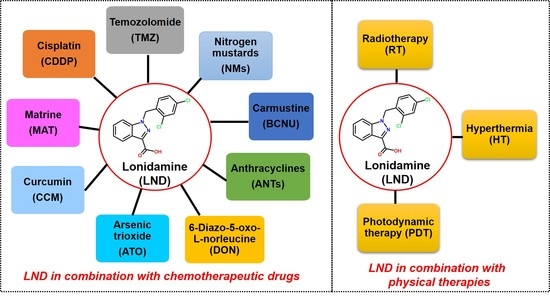
Cancers, Vol. 12, Pages 3332: The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment
|
The p.Ser64Leu and p,Pro104Leu missense variants of PALB2 identified in familial pancreatic cancer patients compromise the DNA damage response
|
Novel homozygous truncating variants in ZMYND15 causing severe oligozoospermia and their implications for male infertility
|
A Novel Variant in COX16 Causes Cytochrome c Oxidase Deficiency, Severe Fatal Neonatal Lactic Acidosis, Encephalopathy, Cardiomyopathy and Liver Dysfunction
|